Welcome
Event Reports
Attended a Socialization “Implementation of Government Regulation No.58/2023”
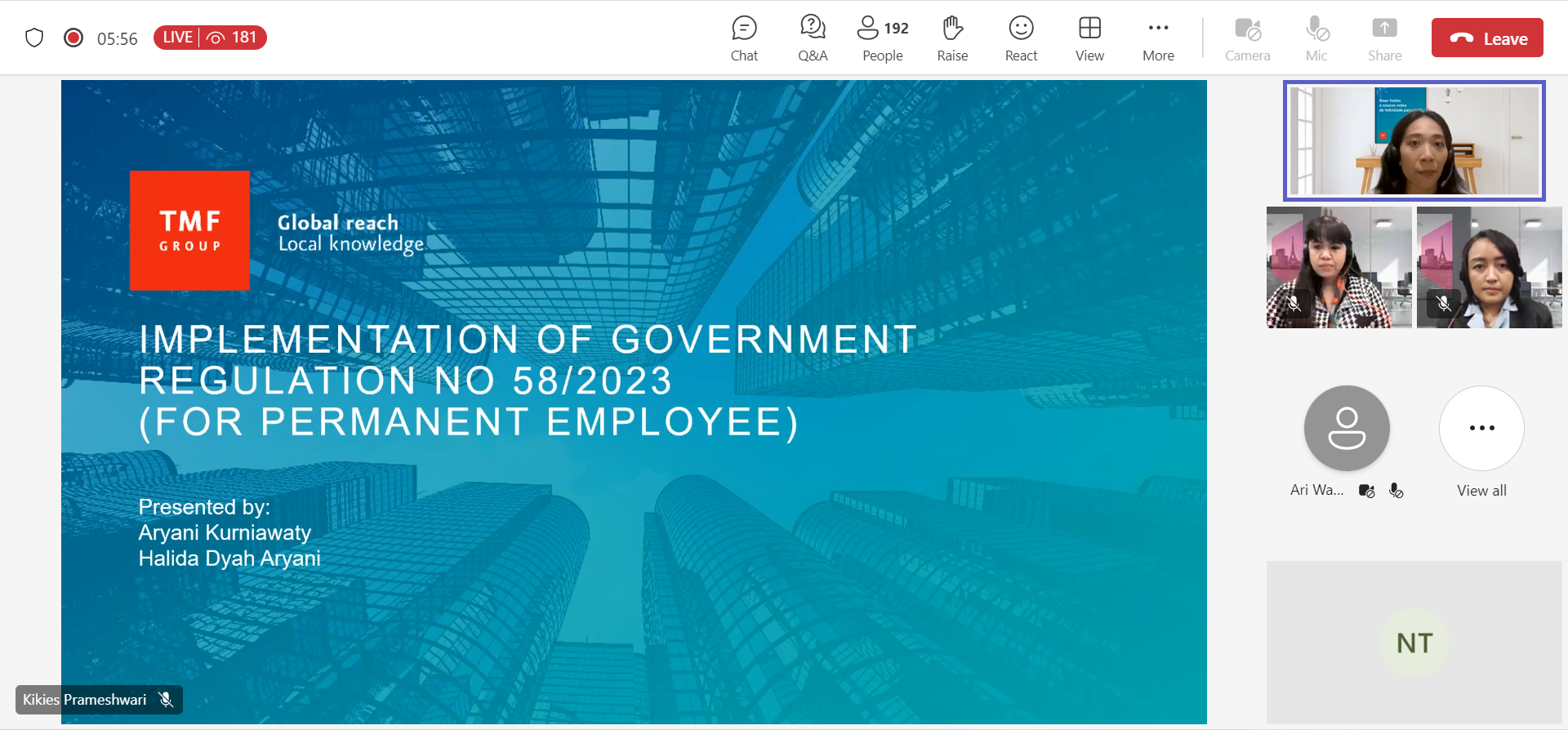
On January 11th, EuroCham was invited to join an online webinar titled “Implementation of Government Regulation No. 58/2023” by TMF Group. Commenced by opening remarks by Muhammad Fauzi Irawan (Country Head of TMF Group Indonesia and Deputy Head of the EuroCham Finance, Tax, and Investment Working Group), this webinar addressed the current regulatory changes on income tax withholding rates and the calculation. This webinar was concluded with a QnA session for the participants. We would like to thank TMF Group for such an insightful webinar!
Introductory Meeting with AmCham ICT Committee
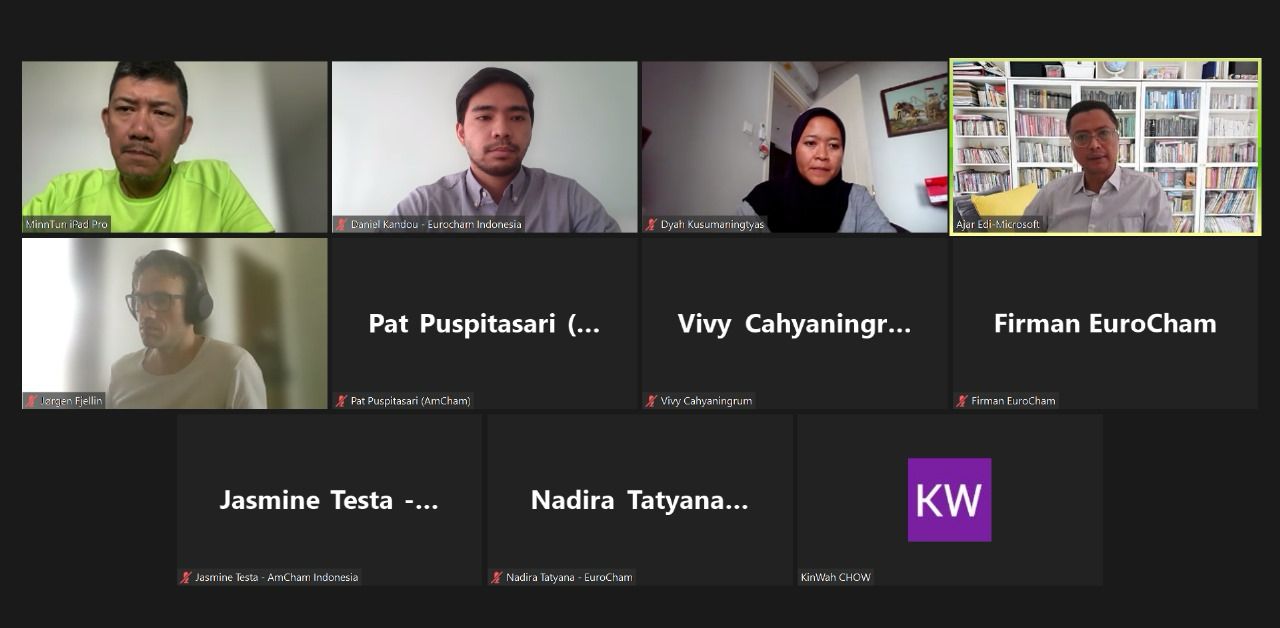
EuroCham’s ICTIP Working Group held an introductory meeting with AmCham’s ICT Committee on 11 January. The meeting discussed potential areas for collaboration in advocacy efforts for 2024, with a focus on the developments of the implementing regulation of the personal data protection law and the establishment of the personal data protection agency; both of which are mandated to be enacted by October 2024. Other issues were discussed as well, such as cybersecurity, artificial intelligence, and others.
The ICTIP Working Group was represented by Minn Tun (Head of the ICTIP Working Group), Jorgen Fjellin (Deputy Head for Technology), and Kin Wah (Deputy Head for Intellectual Property). AmCham ICT Committee was represented by Ajar Edi (Chair of the ICT Committee